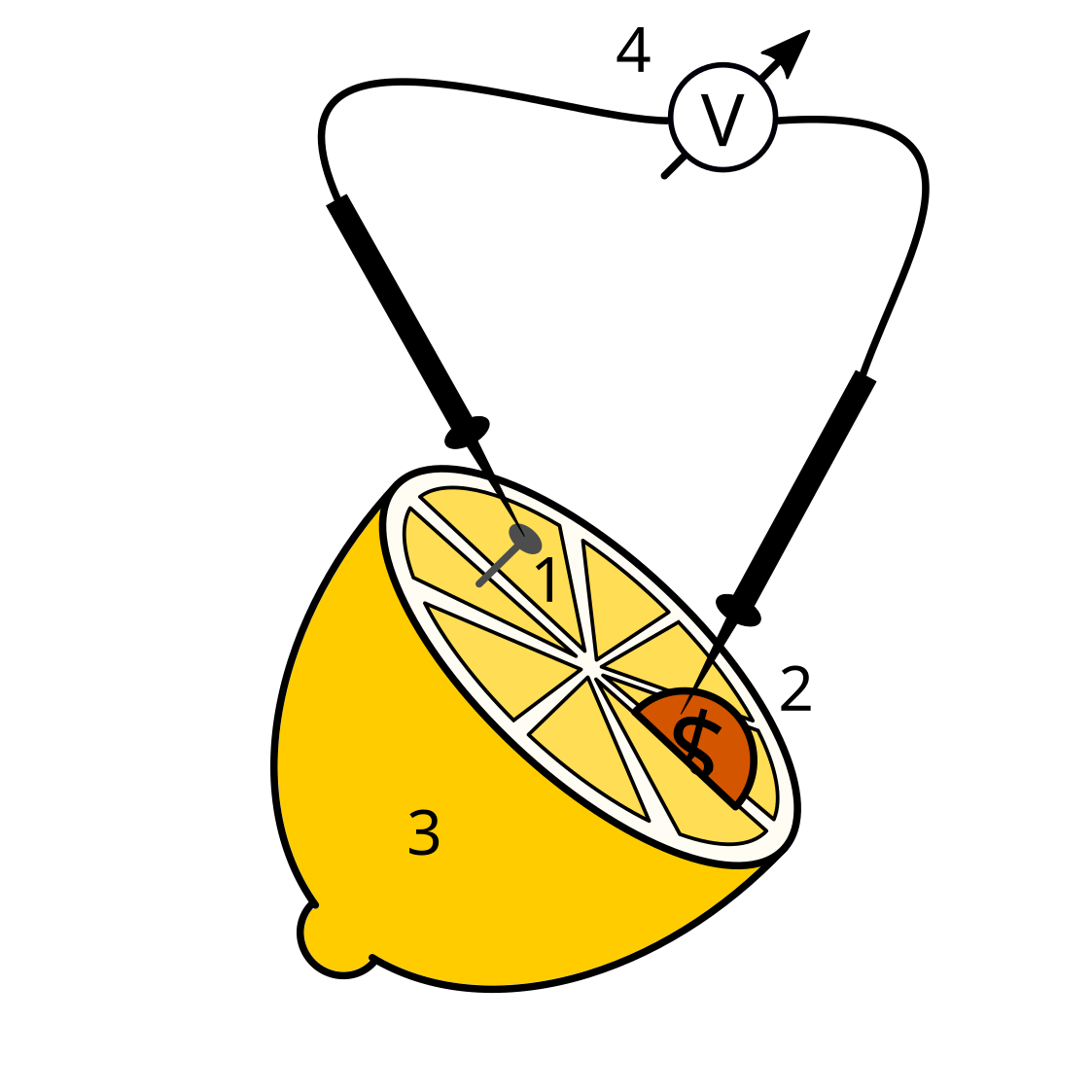
013 – Lemon battery
Fruit battery
potato battery
galvanic element
principle of chemical battery
Introduction to:
Voltage generation, redox reaction, electrochemical potential
Material:
- Galvanized nail or screw / sheet zinc / non-noble metal.
- Coin made of copper / copper sheet / noble metal
- Lemon / fruit (orange, grapefruit, apple, etc.) / potato
- voltmeter / multimeter
+ kitchen knife
Setup:
- Roll the lemon on the tabletop under light pressure so that the pulp is partially destroyed.
- Cut it in half vertically (with regards to the axis through both ends).
- Insert the nail and the coin (“electrodes”) into the lemon a few centimeters apart and connect them to the multimeter.
Procedure:
Measure the electrical voltage between the electrodes, as well as the short-circuit current.
Observation:
A voltage of up to 0.9 V and a current of up to about 0.1 mA can be produced.
Tips:
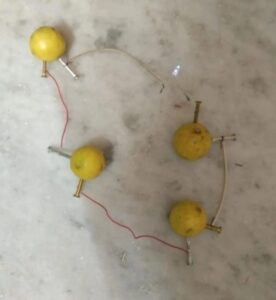
Several lemon batteries can be connected in series. This can be used to operate a light-emitting diode LED.
Field report:
I also told the students to get wire, a 1.5 or 9 volt cell and an LED. For those who didn’t have that, I bought one and gave it to them, and for some of the more enterprising students, I told them to make their own cells with lemon, iron nail and copper wire. Once the battery is ready, they should connect the LED and the two ends of the free wires to different things such as fruit, vegetables, vinegar, cold drinks, water, milk etc and see if the LED lights up. If it does, then the material is an electrolyte.
A big hit with the kids and a headache for the parents.
(from India)